By Gabriele Bedini

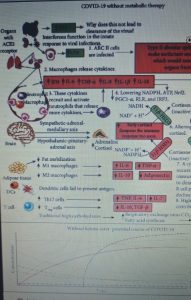
Human SARS-CoV-2 infection is characterized by the fact that some patients develop a broad innate immune response associated with a cytokine storm and acute respiratory distress syndrome (ARDS). This condition is also characterized by an altered redox state, hence oxidative damage and cell death.
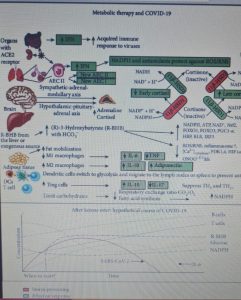
During a virus-induced cytokine storm, metabolic flexibility is impaired due to increased levels of reactive oxygen species (RO ) and reactive nitrogen species (RNS) which damage tissues and downregulate or inactivate many enzymes of central metabolism, including the pyruvate dehydrogenase complex (PDC). This leads to an energy and redox crisis which reduces the proliferation of B and T cells and results in increased cytokine production and cell death. Therefore, therapies that increase (R) -beta-hydroxybutyrate (R-BHB) levels, such as the ketogenic diet or the consumption of exogenous ketones, should restore impaired energy metabolism and redox state (R-BHB activates anti-inflammatory signaling GPR109A and inhibits NLRP3 inflammasome and histone deacetylases).
It is therefore hypothesized that a diet with ketogenic characteristics together with the supplementation of exogenous ketones, administered at the first signs of respiratory distress, increases mitochondrial metabolism “bypassing” the PDC block.
We know that SARS-CoV-2 infects many cell types, particularly type II alveolar epithelial cells (AEC II) in the lungs, in fact SARS-CoV-2 infection occurs particularly in a respiratory syndrome. AEC II has a high energy requirement and relies heavily on the oxidation of fatty acids for energy production. Thus, the partial loss of these functions during infection facilitates viral spread: it disrupts the immune response and tissue repair.
Almost all nucleated cells, including AEC II cells, can recognize the presence of viruses and initiate an innate immune response to recruit phagocytic cells to the site of infection. In a cytokine storm, the number of cells such as macrophages and neutrophils increases along with the levels of pro-inflammatory cytokines, while the number of B and T lymphocytes that are mediators of the adaptive immune response decreases. This results in a non-elimination of the virus and facilitates a positive feedback loop which increases the number of innate immunity cells which secrete additional cytokines. This cytokine storm emerges as a major culprit in acute respiratory distress syndrome (ARDS), multi-organ dysfunction, and patient death in CoViD-19.
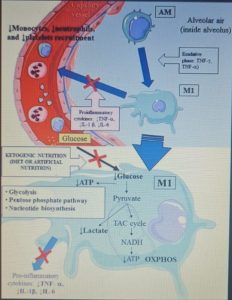
Figure 1 shows the mechanisms leading to acute respiratory distress syndrome and mortality following SARS-CoV-2 infection. While Figure 2 summarizes the proposed mechanisms and time course of SARS-CoV-2 infection when using ketone-based metabolic therapy.
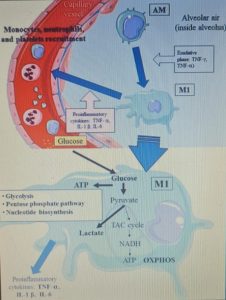
Furthermore, the hyperactivation of M1 macrophages with a pro-inflammatory phenotype, which is linked to aerobic glycolysis, leads to the recruitment of monocytes, neutrophils and platelets from the circulating blood and plays a crucial role in thrombus inflammation (as recently demonstrated) through the formation of extracellular traps of neutrophils and monocyte platelet aggregates, which could be responsible for the DIC disseminated intravascular coagulation that characterizes CoViD-19 .
The modulation of glucose availability for activated M1 macrophages via a eucaloric ketogenic diet (EKD) could represent a possible metabolic tool to reduce ATP production from aerobic glycolysis in the M1 macrophage phenotype during the exudative phase. This approach could reduce the overproduction of cytokines and, consequently, the accumulation of neutrophils, monocytes and platelets from the blood. Second, an EKD could be beneficial for the metabolism of anti-inflammatory M2 macrophages because these cells predominantly express oxidative phosphorylation enzymes and are better fueled by the oxidation of fatty acids in the mitochondria. An EKD could ensure the availability of free fatty acids, which are an optimal supply of “fuel” for these cells. Finally, EKD could also reduce the formation of lactate by macrophages due to glycolysis, in such a way as to favor the production of INF-I (since this would be inhibited by excessive lactate production).
From a metabolic point of view, the activation of the M1 phenotype induces a metabolic shift in the production of adenosine triphosphate (ATP) from OXPHOS to aerobic glycolysis (Warburg effect). The activity of the tricarboxylic acid (TCA) cycle is reduced; whereas lactate production is increased in the presence of hypoxia, glucose overload, or both.
We therefore hypothesize that a eucaloric ketogenic diet (EKD), reducing the dietary oral intake of glucose, can favor the anti-inflammatory process through the modulation of the immune metabolism.
The metabolic modulation induced by a ketogenic diet ( KD ) can affect the following four targets: inhibition of M1 macrophages , activation of M2 macrophages, disinhibition of IFN-I production induced by lactate overproduction, and decreased virus synthesis in cells.
The reduction of glucose uptake by M1 macrophages represents the main goal of the metabolic treatment of CSS, because aerobic glycolysis is the main way in which ATP is produced in activated M1 macrophages and allows these to perform effector functions, such as the production of inflammatory cytokines .
An EKD could reduce the availability of glucose for aerobic glycolysis (Warburg -like effect) in M1 macrophages. Indeed, the main objective of this approach would be to inhibit the hyperactivation of M1 phagocytes, which causes the overproduction of pro-inflammatory cytokines ( IFN-γ, TNF-α and IL-1 β ), leading to an excessive accumulation of monocytes, neutrophils and platelets from the blood. At the same time, EKD could also be beneficial for the anti-inflammatory metabolism of M2 macrophages, because these cells are best fed by the oxidation of fatty acids in the mitochondria. M2 cells (anti-inflammatory) derived from M1 cells, in fact appear in the rehabilitation phase of ALI / ARDS and limit pro-inflammatory cytokines in the alveolar space through the production of anti-inflammatory cytokines ( IL-10 and IL-1 ).
A final metabolic hypothesis in the treatment of CoViD-19 emerges from a recent in vitro proteomics study of SARS-CoV-2 infected host cells, which aimed to detect potential therapeutic targets. It was observed that targeting glycolysis with the glycolysis inhibitor deoxy-d-glucose (a hexokinase inhibitor effective against other cultured viruses) replication of SARS-CoV-2 was inhibited. Since agents that reduce glycolysis activity could be potential therapeutic agents for the treatment of CoViD-19, a similar antiglycolytic effect could be achieved by KD, the possible implications of which are summarized in the table below.
What is more, in obese patients with CoViD-19, a low-calorie KD could also be used, considering the various therapeutic dietary patterns described in the literature. In fact, the term ketogenic diet describes a variety of diets of varying composition of macronutrients such as fat and protein but extremely low in carbohydrates.
EKDs are clinically used in the treatment of other diseases. Many of these diseases have underlying metabolic dysfunctions and chronic inflammation that have been linked to a state of hyperglycemia. There is a consensus that ketosis protects healthy tissues from oxidative stress by simultaneously decreasing ROS production and increasing endogenous antioxidant capacity. But a KD also minimizes blood glucose spikes and reduces circulating inflammatory markers. By raising hydroxybutyrate, KD is able to activate hydroxycarboxylic acid receptor 2 (a G-protein coupled receptor) which inhibits nuclear factor-KB in macrophages.
Although low calorie KD and very low calorie KD are used primarily in the treatment of obesity, carbohydrate restriction is also the most important tool in the management of diabetes and hyperglycemia. KDs offer benefits in this regard. In intensive care units (ICUs), there is a close relationship between glucose at admission and mortality, not only in patients with type 2 diabetes but also in those without a history of diabetes mellitus.
According to the literature, hyperlipidic diets, although non-ketogenic, could be beneficial in ICU patients undergoing artificial ventilation and could also improve respiratory failure.
The change in host metabolism, in particular the change in metabolic state (from carbohydrate-dependent glycolytic to a ketogenic state) must also be considered as it can affect viral replication as well as the impact of intermittent fasting ( IF ) in the activation of the metabolic switch together with the integration with medium chain triglycerides (MCT) such as lauric acid, in repressing the formation of the viral envelope and the replication of virus itself. In fact, the combination of IF and an MCT -rich ketogenic diet is believed to work as a prophylactic measure for healthy people and adjunct therapy for infected people: a ketogenic breakfast diet regime along with supplementing with two doses of acid-rich MCTs. Lauric at breakfast and lunch, followed by 8-12 hours IF, could be a potential prophylactic strategy and adjuvant therapy to fight SARS-CoV- 2 infections .
Initial preliminary data, using the globally designed ecological observational study, suggest a role of dietary intakes and nutritional status in favoring or disadvantaging the body environment necessary for viral growth and replication and indicate the importance of dietary factors in battle against pandemic infection.
Interestingly, dietary modifications, including fasting and calorie restrictions have been proposed as one of the approaches to altering metabolic pathways.
Such metabolic change by carrying out a special diet and exercise pattern is an established strategy that influences the host’s metabolism and can lead to improved health conditions. This is a very safe therapeutic and prophylactic approach that can be easily followed by a wide range of individuals even with varying comorbidities.
REFERENCES:
Oxid Med Cell Longev . 2020; 2020: 6401341.Published online 2020 Sep 9. doi:
10.1155 / 2020/6401341 PMCID: PMC7519203PMID: 33014275 COVID-19:
Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm
Patrick C. Bradshaw , 1 William A. Seeds , 2 Alexandra C. Miller , 3, 4 Vikrant R. Mahajan , 5 and William M. Curtis 1
Nutrition . 2020 November-December; 79: 110967.Published online 2020 Aug 10. doi: 10.1016 / j.nut.2020.110967 PMCID: PMC7416786PMID: 32942131
Induction of ketosis as a potential therapeutic option to limit hyperglycemia and prevent cytokine storm in COVID-19
Samir Giuseppe Sukkar , MDa, ⁎ and Matteo Bassetti , MD, Ph.Db
Ann Nutr Metab . 2020 Sep 18: 1–7.Published online 2020 Sep 18. doi: 10.1159 / 000510508 PMCID: PMC7573915PMID: 32950986
Switching Host Metabolism as an Approach to Dampen SARS-CoV-2 Infection
Sameh Soliman , a MoezAlIslam E. Faris , b, * Zakaria Ratemi , c and Rabih Halwanid